This is a favourite question by any thermal dynamics textbook. :)
First of all, we consider what happens at a phase change. At a phase change, the substance is applying all of the heat received into breaking the bonds, and hence, the temperature does not change. However, when the substance accepts heat and is not at a state change, the substance does increase in temperature. The important temperatures to consider, are of of course the melting point and the boiling point. Of water, we know they are 0ºC and 100ºC respectively.
Note that in these equations, one always is evaluating the difference in temperature. This type of calculation does not require conversion from Celsius to Kelvin as the difference will stay the same. (If you are skeptical, try it out! I only mention this as the question came up in lecture and seemed to be shared by many people.)
So returning to the initial question, how much heat is required?
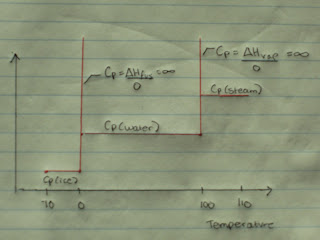
Now, examining the heat capacity of water at constant pressure at the phase changes we see a the following graph. This graph is a simplified version of the real relationship of heat capacity while applying heat to a substance, as the heat capacity between the phase change would not be constant. However, we consider it to be constant to simplify matters.
The intriguing part of the above graph is what happens to the heat capacity at the phase transition. The change in temperature is zero at these places. This can be expressed by:
The heat capacity is infinite at a phase change? What does this mean?
Recall, the system will absorb all of the heat given without changing the temperature, and so until the bonds are broken, it will happily accept ALL the heat provided. The heat capacity is infinite at the phase change, as the system has no limit for receiving heat.


No comments:
Post a Comment