Physical Chemistry Mock Midterm Exam
**disclaimer: I have
no idea exactly what will be on the midterm exam beyond what was listed by the professor in class, although I
do know these section headings are accurate. This mock midterm is written only as
a study tool. It’s usefulness is debatable.
Part I:
Definitions (20%)
Provide definitions for each of the following terms. Do NOT
give any examples of the terms or you will lose marks. (1 mark per definition, -1 mark if
example given)
1) System: the part of the universe of interest
2) Isolated system: system that exchanges neither matter nor
energy with the surrounding
3) closed system: system that exchanges matter but not
energy with the surroundings
4) open system: system that exchanges both matter and energy
with the surroundings
5) surroundings: the rest of the universe, excluding the
system or part of the universe of interest
6) Zeroth Law of Thermodynamics: If A is in thermal
equilibrium with B and B is in thermal equilibrium with C, then A is in thermal
equilibrium with C
7) Thermal Energy: the energy around us in the
translational, rotational, vibrational states
8) Internal Energy: the energy needed to create the system
from atoms, the thermal energy (kinetic energy of motion – moving, rotational
and vibration) and bond energy – the electrostatic energy of the attraction and
repulsion of nuclei, does not include PV work
9) Heat: transfer of energy by conduction, radiation,
convenction; path dependent
10) Work: energy transferred by a force acting on a
distance; path dependent
11) First Law of Thermodynamics: delta U = q + w, where q is
the heat added to the system, and w is the work done on the system, and delta U
is the change in internal energy
12) Reversible Path: a path that depends only on the initial
and final states of the system
13) Nonreversible Path: a path that depends on the method
used to achieve the final state from the initial state
14) State Function: a property whose change is only
dependent on the initial and final states of a system
15) Heat Capacity (also include definition for molar heat
capacity and specific heat capacity): the amount of heat required to raise the temperature by 1K,
dependent on the translational, rotational and vibrational states of the
molecules
molar heat capacity: the amount of heat required to raise
the temperature by 1K per 1 mol of substance
specific heat capacity: the amount of heat required to raise
the temperature by 1K per 1 kg of substance
16) Translational States: this in my humble opinion, is an unfair
question as the mathematics involving this definition go back to particle in a
box and are a bit complicated.
A better question would be: How do you eliminate the
translational states of a molecule?
Answer: By taking a frame of reference.
17) Rotational States: same as angular momentum, denoted by
Jmj where J goes from 0 to infinity, -J < mj < +J
18) Vibrational States: given in integer values from 0 to
infinity
19) Equiparition of Energy: the division of energy amongst
all of the degrees of freedom of the molecule (this definition may be
incomplete, however with the content of the course thus far, and its not
rigorous nature, I suspect it may be ok)
20) Bond Energy: the energy required to break a chemical
bond into its constituents
21) Hess’s Law: state functions do not depend on the path
22) Heat of Formation of a Molecule: the heat required to
form a molecule from its elements
22) Heat of Combustion of a Molecule: the heat required to
burn a molecule (react with gaseous oxygen) to usually produce gaseous carbon
dioxide and liquid water
23) Enthalpy: H = U + PV where U is the internal energy of a
system, P is the pressure of the surroundings, and v is the volume of a system
26) State: the parameters that are measured and describe a
system under those conditions
Part II:
Calculations
Provide all work, including appropriate equations, notation,
and manipulation.
Try (or retry) calculations from the textbook without
looking at the solutions manual.
For a good selection of questions do:
Energy, Heat, Work
3, 4, 6, 10 (this question might require you to do 9 or something, sorry)
Thermochemistry
15, 17, 21, 24, 29, 32, 34
Equiparitioning of
Energy
1)
Calculate the number of degrees of freedom for
carbon dioxide. Estimate the molar heat capacity of the substance.
2)
Calculate the number of degrees of freedom for
methane. Estimate the molar heat capacity of the substance.
Part III: Short
Answers
1)
What type of system is a coffee cup calorimeter?
What type of system is a bomb calorimeter? Compare and contrast.
A coffee cup calorimeter is a closed system. Matter cannot
leave the system, but energy can. A bomb calorimeter is an isolated system, so
both matter and energy cannot leave the system.
2)
If system A is 25ºC and system B is 25ºC, and
system C is in equilibrium with system B, what can we conclude about the
temperature of system C and its relationship with system A?
Using the Zeroth Law of Thermodynamics, we know that since system C is in equilibrium with system B, it must have the same temperature of 25ºC. System C may be in thermal equilibrium with system A if it is in contact with system A, or if it is in contact with system B and system B is in contact with system A. But the problem doesn't include enough information to make a solid conclusion about the relationship of system C and A.
3)
Draw potential energy diagram of a diatomic
molecule. Label the place of greatest repulsion, A. Label the place of greatest
stability, B. Label zero point, C. Label and show the bond energy. Draw in the
largest state (rotational/vibrational/translational) , and discuss the
placement and magnitude of the other states
(rotational/vibrational/translational).
4)
Is the maximum work done by the system
reversible or irreversible? Explain referring to a P versus V diagram.
The maximum work done by the system is reversible. It is
done along the same isotherm (at constant temperature), and depends on only the
final and initial states, not the path. In an isothermal expansion or compression, the process is very slow, and so the work done by or on the system is replaced by the heat into or out of the system for a change of internal energy of zero. (There is a nice diagram on page 58 of the text that you could refer to with this answer.)
5)
Using the definition of enthalpy, show that it
is equal to the heat at constant pressure.
6)
Define both the heat capacity at constant volume
and the heat capacity at constant pressure.
7)
Is work a state function? Explain.
No, work is not a state function, as it is path dependent.
(Using a car, crane or helicopter to move an object all takes different amounts
of energy to reach the final state)
8)
Discuss how differential calorimetry finds the
unknown heat capacity of a substance.
Differential calorimetry compares a sample of unknown heat capacity with a sample of known heat capacity called the reference sample. This reference must be well-defined over a large range of temperatures, and not undergo a phase transition. Then you test the two samples. If the unknown undergoes a phase transition, a peak on a heat versus temperature graph will appear. Comparing this value with the value of the known heat capacity of the reference sample will aid in calculating the heat capacity of the unknown. qs - qr
9)
What is the standard enthalpy of formation of
oxygen gas?
As oxygen gas is in elemental form, its standard enthalpy of
formation is zero.
10)
Why
is the bond energy always positive?
The bond energy is the amount of energy required to break a
molecule into its constituents. Energy is usually required to put into the
system (positive) to break a chemical bond.
11)
Hydrogen gas only has 2 rotational degrees of freedom. Explain
why.
Hydrogen gas can rotate along the three axes. However, the x
and y axes exhibit much greater rotation for a linear molecule as does the z
axes, since the x and y axes contain the two atoms whereas the z axis does not
and so is not as greatly effected.
12)
Discuss the effects of temperature when comparing calculated
heat capacities using equiparitioning of energy and the actual measured heat
capacity values.
At warmer temperatures, there is greater agreement of the
calculated heat capacities with the actual heat capacity values. At colder
temperatures there is less agreement between the calculated and actual heat
capacities. This is because the vibrational molecules are more widely spaced
and energy cannot move as freely between vibrational modes, so a transition requires
a more significant (or sufficiently large quantum) amount of energy.
Part IV: True or
False
This section will NOT
be on the exam, however, it may provide a good snapshot as to whether the
definitions are understood.
1)
Heat leaves the system. q is positive. True/False q is negative.
2)
Energy is stored in chemical bonds. True/false
energy is stored in the degrees of freedom of a molecule
3)
The system does work. w is negative. True/false
4)
Internal energy does not depend on PV work. True/false
5)
Bond energy is usually greater than thermal
energy. True/false
6)
A diatomic gas has 2 degrees of translational
energy. True/false
A diatomic gas has 3 degrees of translational energy and 2 degrees of
rotational energy.
7)
PV work is positive for an expansion of a gas. True/false
PV work is negative for the expansion of a gas and positive for the
compression of a gas.
8)
The lowest vibrational state is at the zero
point. True/false The lowest vibrational state is
slightly above the zero point.
9)
The heat capacity at a phase change is infinite. True/false
10) Vibrational states are the same as angular momentum. True/false
Rotational states are the same as angular momentum.
Part V: Bonus
Questions
1)
Show that PV=nRT is a state function using
partial derivatives and Euler’s Theorem for Exactness without referring to
Appendix C. (Just kidding! Bad joke I know.)
Refer to post titled “Friday Night State Functions.” But seriously we don't have to know this.
2)
Describe your experience and opinion of the
textbook with regards to whether you have the e-book or hardcopy, its
acquisition and availability, its use as a study tool, and to what level it
corresponds to and complements the lectures.
I have heard many (at least 5 opinions not my own) nuanced
responses to this question, and they all have strikingly similar themes.



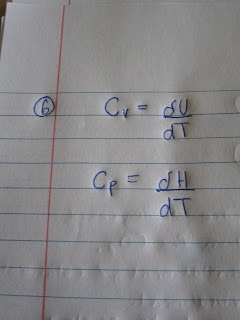
Wow, thank you so much! Good explanations!
ReplyDeleteThank you. :) I really hope there aren't mistakes.
ReplyDelete